Beyond the Colonoscopy: Navigating the New (and Complex) Landscape of Colorectal Cancer Screening
- OliveHealth

- Dec 11
- 3 min read
by Dr. Ed Fuentes
Guidelines have shifted to age 45, and new blood tests are entering the market. Here is a data-driven look at the trade-offs between efficacy, prevention, and convenience in CRC screening.
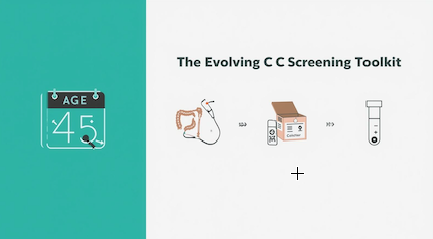
The Evolving Colon Rectal Cancer Screening Toolkit
Colorectal cancer (CRC) screening is undergoing a significant transformation. With incidence rates rising in younger adults, major organizations like the USPSTF and the American Cancer Society now recommend screening begin at age 45 for average-risk adults.
For years, the prevailing maxim among primary care physicians has been: "The best screening test is the test that gets done."
This pragmatic approach acknowledges that resistance to the "gold standard" colonoscopy is real. But with the arrival of new high-tech stool tests and highly anticipated blood-based biomarkers, the conversation is becoming more complex.
Are all tests created equal? Is convenience being prioritized over prevention? Here is a professional review of the current landscape based on recent clinical data and guidelines.
The Fundamental Trade-Off: Prevention vs. Detection
When evaluating screening options, it is vital to distinguish between two different goals:
Prevention: Finding and removing pre-cancerous polyps before they turn into cancer.
Detection: Finding cancer that already exists at an early, treatable stage.
While all recommended tests can detect cancer, their ability to prevent it varies drastically.

The Comparison of the CRC Screening Tool Kit
A Closer Look at the Options
1. The Gold Standard: Colonoscopy
Colonoscopy remains the only tier-one strategy that is both diagnostic and therapeutic. A gastroenterologist can find and remove pre-cancerous lesions during the same procedure. Its sensitivity for both cancer and advanced polyps is near perfect in optimal conditions.
The friction: It requires arduous bowel preparation, sedation, and time away from work.
2. The Established Alternatives: Stool-Based Tests
a. These at-home tests are non-invasive but must be done more frequently.
FIT (Fecal Immunochemical Test): Done annually. It detects hidden blood. It is highly specific (low false-positive rate) and very cost-effective.
b. mt-sDNA (e.g., Cologuard): Done every 3 years. It detects blood plus DNA markers. It has higher sensitivity for detecting existing cancer than FIT (approx. 92%), but a higher false-positive rate, meaning more people are sent for unnecessary follow-up colonoscopies.
3. The New Frontier: The Blood Test Option
The recent emergence of blood-based tests, such as Guardant Health Shield, has generated significant excitement. The promise of screening via a simple blood draw during a routine physical is alluring.
However, recent prospective studies highlight critical limitations. While the Shield test demonstrated strong sensitivity for detecting existing Stage I-III cancer (87.5%), its sensitivity for detecting advanced precancerous lesions was only 13%.
Furthermore, specificity was roughly 90%, meaning about 1 in 10 healthy participants received a false-positive result.
The "Second-Line" Consensus
Due to the inability to effectively detect pre-cancerous polyps, multidisciplinary expert panels currently view blood tests not as a replacement for established screening, but as a second-line option. They should primarily be offered to the approximately 3 in 10 adults who decline colonoscopy or stool-based testing.
Although the blood test is not the gold standard for CRC, its diagnostic value outweighs the risk of skipping screening altogether

The Financial Nuance: The "Diagnostic Loophole"
For employers and patients, understanding insurance coverage is critical. Under the ACA, recommended screening tests are covered with no out-of-pocket costs.
However, confusion often arises when a non-invasive test (stool or blood) comes back positive. This triggers a mandatory follow-up colonoscopy. Historically, some insurers classified this follow-up as a "diagnostic" procedure rather than a "screening," subjecting the patient to deductibles and co-pays.
While recent rule changes have largely closed this loophole for Medicare and many private plans, ensuring that the follow-up colonoscopy is also covered at zero cost, patients must still be vigilant and verify coverage with their provider.
The Bottom Line for Healthcare Leaders
The expansion of the screening toolkit is a positive development for public health, but it requires more nuanced conversations in primary care. Convenience is a powerful driver of adherence, but it comes at the cost of prevention efficacy. The goal remains getting eligible adults screened, but ensuring they understand the strengths and limitations of the test they choose is now more important than ever.


Comments